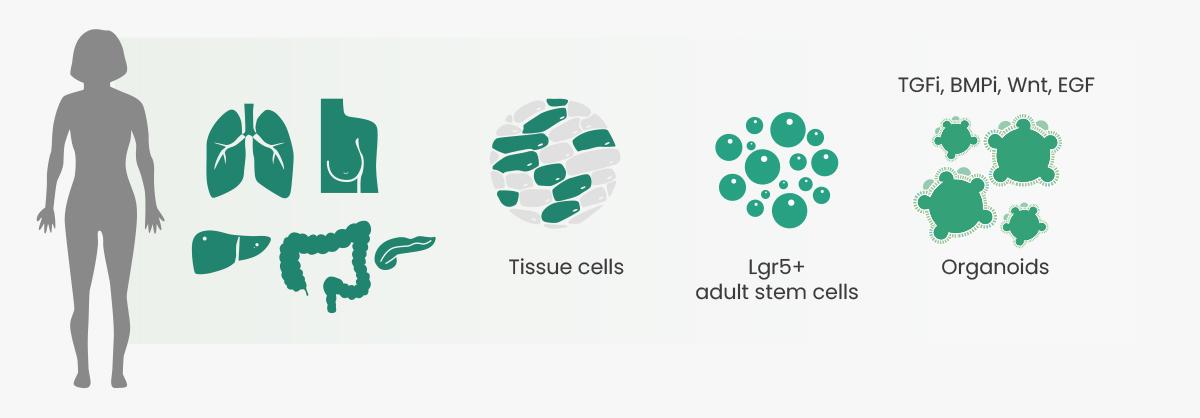
In the field of in-vitro 3D culture technologies, specifically organoids, recent advances have revolutionised the development of more physiologically relevant human cancer models. These models are crucial for translating basic cancer research into effective treatment strategies for patients with cancer. One significant advantage of organoids is that they can be cultured from healthy tumor tissues obtained from individual patients. This approach enables physicians to conduct patient-specific drug testing, the results of which support development of personalised treatment regimens.
Traditionally, cancer-drug screening has relied on two-dimensional cell-culture models and patient-derived tumor xenograft (PDX) models. Although these models have their advantages, they fail to capture the complexity and heterogeneity of tumors in a three-dimensional context. Moreover, PDX models are expensive and time-consuming, which limits their utilisation. The development of tumor organoids has revolutionised cancer research by providing more physiological and personalised models for cancer research. Given the ability of these models to mimic the tumor microenvironment, their potential for patient-specific drug testing, and their advantages over traditional models, organoids have become a promising approach to improved cancer-treatment strategies.
Breakthroughs for the Use of Organoid Models
In 2009, Sato et al. described the ability to indefinitely culture 3D organoids in Corning Matrigel matrix for organoid culture (Corning Inc., US) from single Lgr5-positive intestinal stem cells under defined culture conditions that artificially provide niche factors (e.g., R-spondin, noggin, epidermal growth factors). Intestinal organoids were successfully established, and the next five years saw the establishment of numerous organoid culture protocols, such as for the colon and small intestine, retina, brain, liver, stomach, and breast. In 2014, the research of Gao et al. and Li et al. led to breakthroughs for using organoid models in cancer research. The researchers performed comprehensive genomic analysis, which showed that the organoids closely recapitulated in vivo prostate cancers in copy-number alterations, gene-expression subtypes, and histological patterns. Subsequently, researchers successfully constructed colorectal cancer, gastric cancer, prostate cancer, bladder tumors, esophageal cancer, endometrial cancer, and other tumor organoid models.
Cell-Culture Models
Compared with traditional tumor research models, tumor organoids can more accurately reflect the tumor microenvironment. They not only can intuitively show the tumor growth process, but also reflect individual differences in patients. Other advantages include closer to physiological cells, stable genome, and suitability for biological transfection and high-throughput screening (Table 1). Overall, tumor organoids provide an organ-level research system that is intuitive, reliable, efficient, and avoids ethical disputes.
Importance of Cytokines in Organoid Culture
Successful culture of organoids requires specific items, such as Matrigel matrix and culture media, as well as essential factor cytokines. The categories of these cytokines mainly include activators, inhibitors, and hormones that promote cell growth, differentiation, and signaling pathways; cytokines that promote cell proliferation; and cytokines added to improve the success rate of organoid culture. Different organoid cultures survive in medium supplemented with growth factors relevant to the organ of interest. For example, mouse small-intestinal organoid cultures survive in medium supplemented with the growth factors noggin, R-spondin 1 or R-spondin 3, and epidermal growth factor (EGF), and the addition of exogenous Wnt is often not needed because Paneth cells in culture produce sufficient Wnt for organoid proliferation.
As a global leading supplier of high-quality recombinant cytokines, Sino Biological has developed a panel of recombinant growth factors with high bioactivity, batch-to-batch consistency, and purity to enable optimal and consistent organoid growth. The applications of these growth factors in organoid culture are shown in Table 2.
Featured Recombinant Growth Factor Proteins
Organoid Applications in Cancer Research
Organoids have become an indispensable 3D model in cancer research because they can recapitulate many aspects of the complex structure and function of the corresponding tissue in vivo. Application of organoids should consider the tumor organoid biobank, new drug screening, and precision medicine.
Tumor Organoid Biobank
The tumor organoid biobank can be used to perform drug sensitivity testing, drug screening, and toxicity testing based on tumor heterogeneity. Specific cancer examples include breast cancer, which is a highly heterogeneous cancer containing multiple pathological subtypes. Sachs et al. established 95 breast cancer organoids from 155 breast cancer specimens, with a success rate >80%. The distribution of pathological types in this organoid biobank is consistent with the epidemiology of breast cancer, including 50% to 80% invasive ductal carcinoma and 5% to 15% invasive lobular carcinoma. In addition, whole-genome sequencing and transcriptome sequencing have revealed that the copy-number variations and gene-expression profiles of organoids were highly similar to those of the parent tumors even after long-term culture.
Glioblastoma is the most common brain tumor in adults, is highly aggressive, and exhibits strong resistance to target and immunotherapy. Jacob et al. established 70 organoids from 53 patients with glioblastoma. Additionally, transcriptome, whole-exome, and single-cell transcriptome sequencing have shown that glioblastoma organoids largely retain intra- and inter-tumor heterogeneity. On this basis, the team co-cultured glioblastoma organoids with chimeric antigen-receptor T cells, explored the response to CAR-T-cell therapy, and revealed the application potential of tumor organoids in immunotherapy.
New Drug Screening
Organoids are ideal models for drug screening. These immortalised cells have unlimited proliferation capacity, but they usually fail to represent the phenotypes and lose genetic heterogeneity of the original tumor during long-term culture, which may increase the failure rate of clinical drug-screening trials. Hans Clevers et al. established a patient-derived colorectal tumor organoids biobank for the first time corresponding to normal tissues and used high-throughput drug screens (including 25 clinical drugs and 10 chemotherapy drugs) to analyze 83 drug combinations. There are 29 drugs in clinical trials and 29 compounds targeting different cancer targets, and it was found that cetuximab is not sensitive to organoid responses in patients with BRAF mutations and that Nutlin-3a is sensitive to organoids with TP53 gene mutations. Consistent with the results of a previous retrospective study of clinical patients, Wnt secretion inhibitors were also found to have a potential role in patients with mutations in the negative Wnt feedback regulator of RNF43.
Precision Medicine
Organoids have shown great potential in the field of precision medicine, which aims to tailor medical treatments to individual patients on the basis of their specific genomics and metabolomics. By providing a more physiologically relevant and personalized model of human organs or tissues, organoids can be used to predict individual patient responses to drugs and other treatments. This approach makes improvements in assay speed, reproducibility, standardization, and automation, which are necessary to realize the translational potential of PDOs as clinical tools.
Conclusion
Considering some of the major limitations in 2D cell cultures and animal models, there is no doubt that 3D organoid systems can provide new opportunities for developing organ mechanisms that can address important challenges in biomedical industries. Organoids also hold great promise for many translational applications, such as regenerative medicine and gene editing, thereby revolutionizing in vitro culture tools for biomedical research. However, several obstacles remain in development of 3D organoids that need to be overcome, including regulating self-organization to generate developed organoids, reducing the high diversity of organoid cultures, increasing the physiologically relevant shapes and sizes, prolonging organoid lifespan to create matured organoids, and recapitulating the biological complexity of native organs by incorporating the major biological compartments, such as vasculature and nervous systems. Overcoming these remaining obstacles will eventually lead to more approved organoids for use in clinical trials.










